Platform & Pipeline
The Future of Medicine
Imagine an Ideal Next-Generation Therapeutic Modality
Specificity
selective binding to the target; no non-specific tissue binding
Safety
no harmful breakdown products; non-immunogenic
Developability
well behaved in vitro and in vivo; simple to formulate and administer
Patentability
protect from potential competitors, fast followers and biosimilars
Versatility
high tissue penetration, and ability to precisely control serum half life and in-tissue residency
Modularity
plug-and-play multivalent constructs or chemical conjugation
Stability
able to withstand high temperature, solvent, acid, proteolysis, denaturants, etc.
Affordability
low cost manufacturing using existing infrastructure
Most blockbuster drugs satisfy only a fraction of these attributes
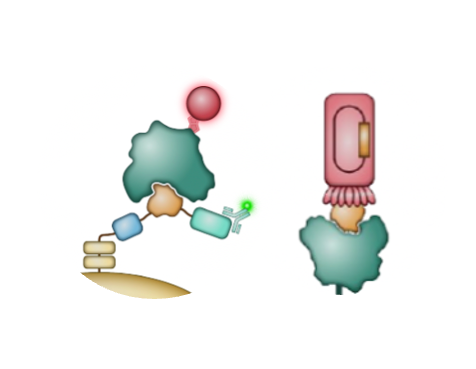
Miniproteins are an ideal therapeutic modality
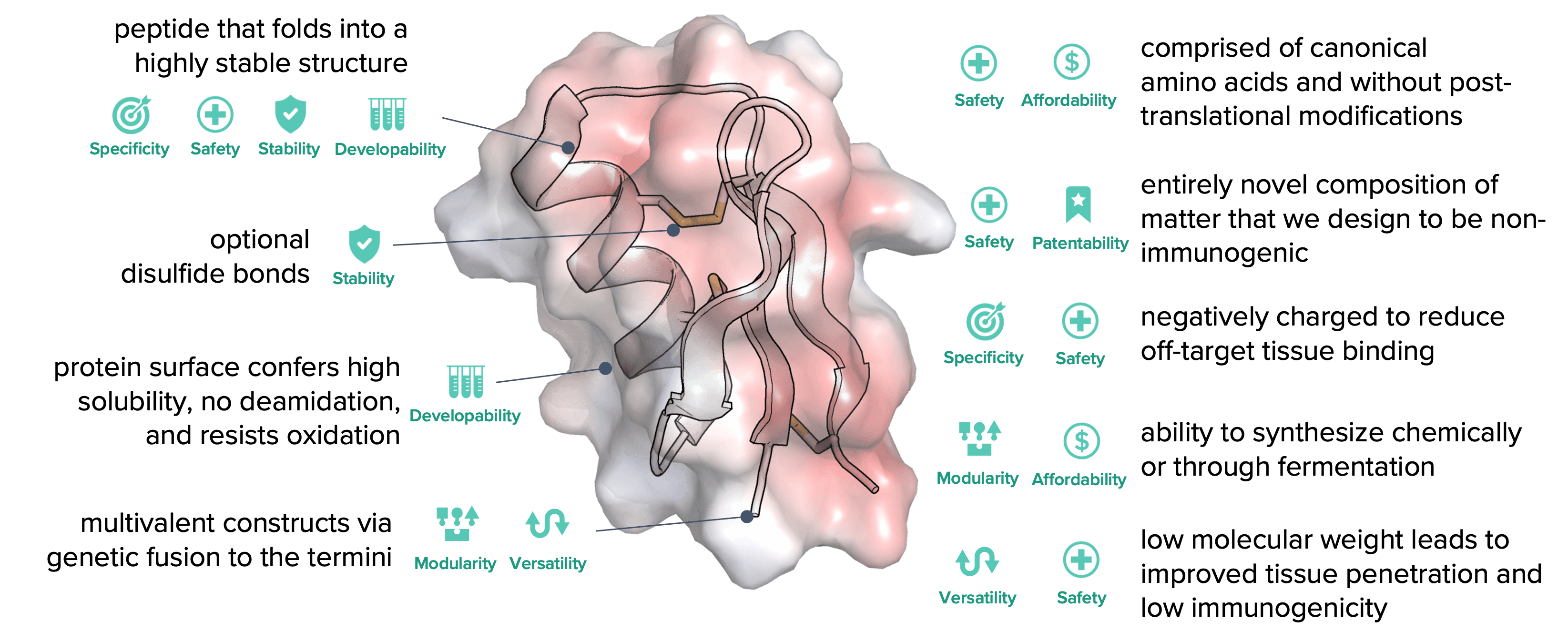
Miniproteins are an ideal therapeutic modality
peptide that folds into a highly stable structure
optional disulfide bonds
protein surface confers high solubility, no deamidation, and resists oxidation
multivalent constructs via genetic fusion to the termini
comprised of canonical amino acids and without post-translational modifications
entirely novel
composition of matter that we design to be non-immunogenic
negatively charged to reduce
off-target tissue binding
ability to synthesize chemically
or through fermentation
low molecular weight leads to improved tissue penetration and lack of immunogenicity
From Concept to Candidate
We engineer miniproteins ready for preclinical development in 3-12 weeks

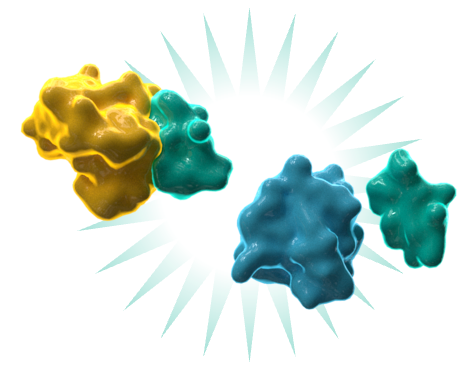
de novo design of miniprotein binders

yeast & phage
display


automated production
& biophysics

“AWESSM”
deep saturation mutagenesis

in silico design

de novo design of miniprotein binders
- Unique “pre-designed” libraries accelerate discovery of hits with ideal drug-like properties
- Targeted design enables conformationally selective binding to a desired epitope; especially helpful for GPCRs
Screening & Discovery

yeast & phage display
- Because our miniproteins are genetically encodable, we’re able to use yeast and/or phage display to screen libraries >109
- Yeast display allows for more precise selection campaigns, while our automated phage method enables massive parallel screening capacity
Characterization

automated production & biophysics
- Our highly-automated protein production and purification platform has the capability to make ~1,000 miniproteins per week
- We perform biophysical characterization in high-throughput, including circular dichroism spectroscopy to measure structure and stability, and surface plasmon resonance to measure binding activity
Optimization

“AWESSM” deep saturation mutagenesis
- The ability to optimize hits into leads is a longstanding challenge in drug development, as it requires the ability to predict and test many variants, often empirically
- We solved this challenge in a novel way by combining artificial intelligence and synthetic biology, creating a highly differentiated method that rapidly yields optimized miniproteins while also producing vast amounts of useful data
Lead Molecules

- We have the ability to design, discover and optimize miniproteins for multiple targets in parallel
- Because drug-like properties are designed into the miniproteins from the start, every molecule with optimized activity is ready to begin pre-clinical studies
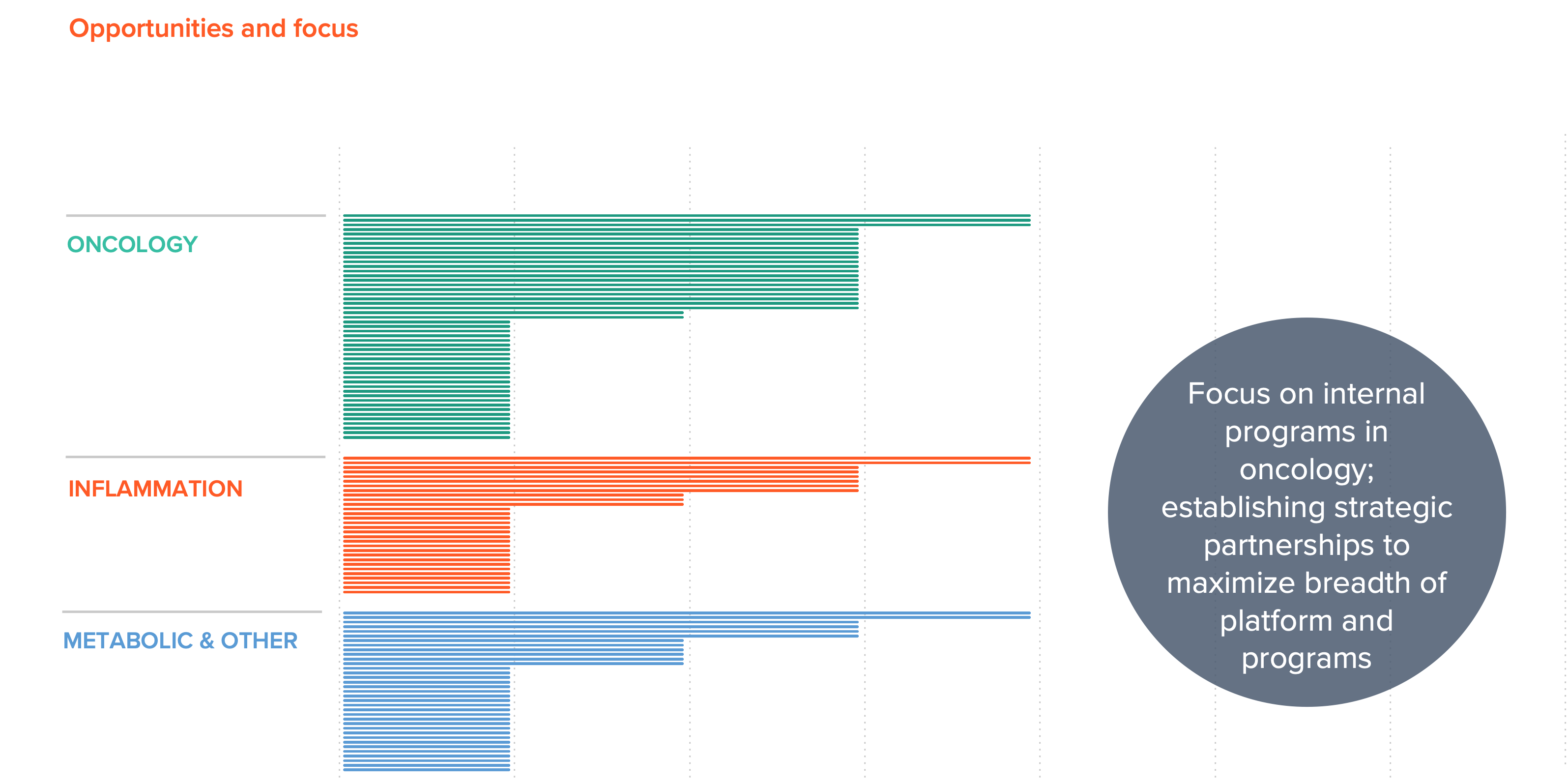
Advancing a Broad and Deep Pipeline
Our proprietary drug discovery engine has already enabled us to validate hits against >100 targets with unparalleled speed. We have in vivo proof of concept data with novel candidates against a number of high value therapeutic targets, and we are currently advancing our own pipeline in oncology while exploring partnerships in inflammation, metabolic diseases and other therapeutic areas to realize the full value of our innovations.